The association between systemic inflammation, lung function and respiratory symptoms in the BOLD study in Northern Europe
In this study, we found significant associations between circulating markers of systemic inflammation, lung function and respiratory symptoms in a general population cohort from Northern Europe, with different patterns according to smoking status. The main findings are summarized in Fig. 3.
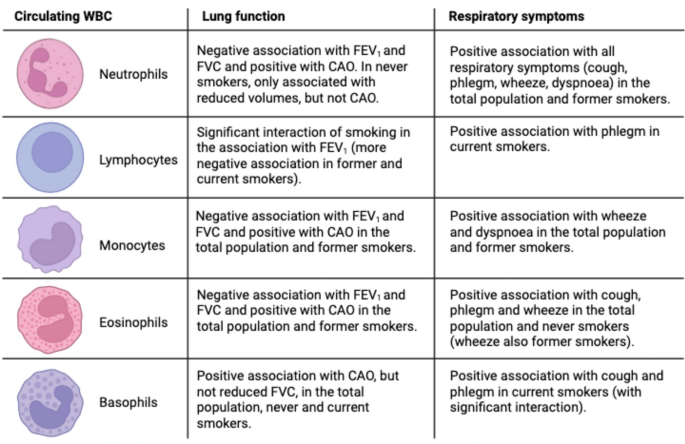
Summary of the findings of the study: associations between inflammatory markers and outcomes (lung function, respiratory symptoms), stratified by smoking status. Image created with BioRender.com. Abbreviations: WBC = white blood cells, FEV1 = forced expiratory volume in the 1 st second, FVC = forced vital capacity, CAO = chronic airflow obstruction, defined as post-bronchodilatory FEV1/FVC values below 5th percentile.
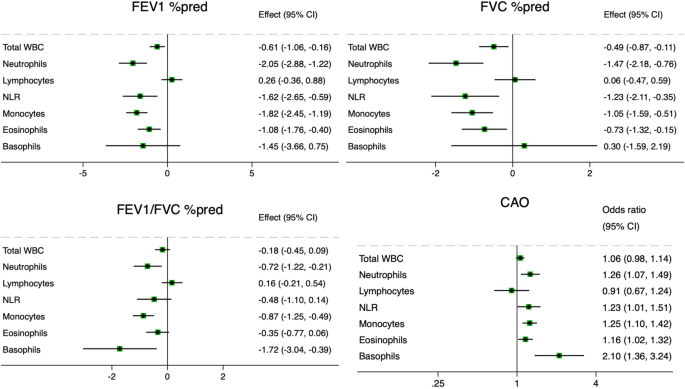
Elevated blood neutrophils were associated with all respiratory symptoms (cough and phlegm – both without a cold and chronic –, wheeze, and dyspnoea), reduction of all lung function indices (FEV1, FVC, FEV1/FVC) and presence of CAO. This is in keeping with the established role of neutrophilic inflammation in the pathogenesis of obstructive lung disease, primarily COPD27, but also subtypes of asthma9. Lewis et al.11 also found an association between blood neutrophil levels and persistent cough, phlegm, dyspnoea, and reduced FEV1, but not wheeze, in a British general population sample. Nerpin et al.5 found an inverse relationship between blood neutrophils and pre-bronchodilatory FEV1, FVC, FEV1/FVC in the NHANES 2007–08 and 2009–10 surveys. When stratified by smoking status, the associations of neutrophils with reduced FEV1 and FVC – but not airflow obstruction – persisted in never-smokers. This suggests that neutrophilic inflammation can be related to lung restriction independently of cigarette smoking. Restrictive spirometric patterns are known to be related to metabolic syndrome, diabetes and obesity, with evidence that the reduction in lung function may precede the other manifestations28. The pathogenetic link is thought to be systemic inflammation, although the exact mechanisms are unknown28. In metabolic diseases, neutrophils release enzymes like myeloperoxidase and neutrophil elastase which promote insulin resistance and inflammation29. It is possible that these mechanisms are also implicated in the pathogenesis of low lung function.
Elevated blood eosinophils were associated with cough and phlegm in the absence of a cold, wheeze, reduced FEV1, reduced FVC, and presence of CAO. These findings also align with Lewis et al.11, although their study also evidenced an association with persistent cough. The association between eosinophils and wheeze in our study was present both in never and former smokers, while the association with reduced lung function was found only in former smokers, and the one with cough and phlegm only in never smokers. This might reflect the multiple aetiologies behind the findings, such as asthma, COPD, their overlap, infections, and potentially other conditions. It is well established that eosinophilia is related to type-2 inflammation, with eosinophils produced in response to IL-5 and further regulated in response to IL-4 and IL-1330. Type-2 inflammation is a feature of asthma and of a subset of subjects with COPD, although this role is more controversial31. There can be multiple differences in the nature of type-2 inflammation between asthma and COPD, such as the type of inflammatory mediators, the role of IgE in mast cell activation and the pathophysiology of mucus hypersecretion32. In our study, it is possible that the association between eosinophils and reduced lung function in former smokers might reflect type-2 inflammation in COPD, while the association with cough and phlegm in never smokers might reflect type-2 inflammation in asthma.
We also evidenced the association of circulating basophils with wheeze, dyspnoea, CAO and reduced FEV1, but not FVC. This might suggest that basophils are involved in airflow obstruction, but not restriction. The association with CAO was present both in never and current smokers, although the significance of the latter was affected by the small sample size. In current smokers, basophils were also related to cough and phlegm. Lewis et al.11 also evidenced significant associations between circulating basophils and wheeze, dyspnoea, and reduced FEV1, but not cough and phlegm, although their study did not stratify for smoking status. Only a few studies have explored the role of basophils in COPD and asthma. Winter et al. found that a mast cell/basophil gene signature measured in sputum was associated with eosinophilic inflammation and lower lung function in severe asthma33 and COPD34. Jogdand et al.35 described both eosinophils and basophils in histological lung COPD samples, with tissue density that increased with disease severity. Mast cells and basophils originate from common precursors. Recent studies hypothesized that mast cells could be a link between COPD and asthma36,37. It is possible that basophils may be related to airflow obstruction in never smokers in our study with IgE-dependent mechanisms typical of asthma32, while they may be related to airflow obstruction and mucus hypersecretion in current smokers with IgE-independent mechanisms typical of COPD38.
We found associations of circulating monocytes with wheeze, dyspnoea, reduced FEV1, reduced FVC and CAO. In stratified analyses, the association with reduced lung function was more evident in former smokers. Similarly, Halper-Stromberg et al.39 reported cross-sectional and longitudinal associations of monocytes with reduced FEV1 in the COPDGene and ECLIPSE cohorts, which consist of former and current smokers. Other studies confirmed associations of increased monocytes with reduced lung function in population-based samples11,12 and also reported associations with persistent cough and phlegm11. Blood monocytes can be recruited into the lung and differentiate into alveolar macrophages, key cells in COPD pathogenesis, which are further activated by cigarette smoking amplifying the inflammatory process40.
We did not find significant associations between blood lymphocyte counts and lung function in the total population, which is in line with the study of Lewis et al.11. However, we found a significant association between increased lymphocytes and phlegm in current smokers, confirmed by a significant interaction. Smoking status also modified the association between lymphocytes and FEV1, with more severe lung function impairment in former and current smokers. This might support a role for increased blood lymphocytes in COPD pathogenesis, although it has also been reported lower cross-sectional FEV1 and greater FEV1 decline in current and former smokers with baseline lymphopenia39. One potential explanation for this difference could be the severity of the disease, as the mean FEV1 in the COPDGene and ECLIPSE cohorts39 was much lower than in our study. Our study also examined the NLR, an index that can reflect inflammation associated with neutrophilia and impaired immune response associated with reduced lymphocytes10,26,41. The NLR has been proposed as a marker of activity and severity of COPD and a predictor of COPD exacerbations. In our study, the NLR showed a profile of association with lung function and respiratory symptoms substantially in line with that of neutrophils.
At present, only blood eosinophils are used as a biomarker of respiratory disease in clinical practice, specifically in the diagnosis and management of COPD42 and asthma43. Our study confirmed their relationship with reduced lung function and presence of respiratory symptoms (cough, phlegm, wheeze). In addition, our study showed a significant association between circulating basophils, lung function and respiratory symptoms, which to our knowledge has been described before only in a British population cohort11. In particular, they were related to airflow obstruction in the whole population, and to cough and phlegm in current smokers. We hypothesize that they might be helpful in differentiating, together with eosinophils, type-2 inflammation in asthma and COPD based on IgE-dependent and Ig-E-independent mechanisms, respectively. This possibility should be confirmed in future studies with other demographics and geographical settings, as well as mechanistic studies.
Our study also suggested that different markers of systemic inflammation may be related to different types of lung function impairment. We observed that circulating neutrophils were related to both airflow obstruction and reduced lung volumes (FEV1 and FVC) in the total population and in former smokers. In never-smokers, they were related to reduced FEV1 and FVC, but not CAO. Accordingly, they could reflect both obstructive and restrictive respiratory disease. The hypothesis of neutrophilic systemic inflammation linked to lung restriction would need to be confirmed in future studies using static lung volumes to assess restrictive impairment. Blood eosinophils and basophils, on the other hand, appeared to be related to CAO and reduced FEV1 to a larger extent than FVC, supporting a predominant role in airflow obstruction rather than restriction.
The strengths of our study include the general population sample, the use of standardized protocol and questionnaires across the study sites and the conduction of spirometry by trained and certified technicians. In addition, all spirometry curves were visually inspected centrally, and only those that passed quality control were used in this study. Compared to previous studies, our study investigated a larger number of outcomes (multiple lung function variables and respiratory symptoms) and it was multi-center, which increases generalizability. Our study also has limitations, including self-reporting respiratory symptoms, which may be affected by recall bias, and the fact that the mean age of our population was 68 years, which impedes the extrapolation of our findings to younger age groups. However, the demographics of the population may provide unique insights on the relationship between systemic inflammation and respiratory health in older adults on whom there is little data. The cross-sectional design does not allow us to infer causality; longitudinal studies are needed to establish temporal relationships between the variables involved. We did not have allergic sensitisation data on the participants, which would have been useful to consider in the current study. The proportion of current smokers in this population was low and this might have affected the significancy of the results. We focused on certain markers of systemic inflammation because we were interested in the individual contribution of WBC sub-populations, but other markers such as the systemic immune inflammation index (SII), the systemic inflammation response index (SIRI), or the aggregate index of systemic inflammation (AISI) may be worth exploring in future studies44.
In conclusion, larger counts of different WBC sub-populations are associated with different lung function indices and respiratory symptoms in the general population. Novel findings of our study include an association of blood basophils with CAO in the general population and with cough and phlegm in current smokers. Together with eosinophils, they might be useful in differentiating the type of inflammation in asthma and COPD. Blood neutrophils were related to reduced FEV1 and reduced FVC – but not CAO – in never smokers, suggesting a potential pathogenetic role in restrictive lung disease. These findings warrant further investigation in future studies.
link







